The synthetic utility of the cope elimination is comparable to the Hofmann elimination of quatenary ammonium hydroxides, but takes place at lower temperatures. Expressions and Identities, Direct For example: This trend is most likely due to the fact that the less highly substituted β-carbon bears more hydrogen atoms than the more highly substituted one; at a given moment, in a sample of the substrate, there are more molecules in which a hydrogen atom on the less highly substituted beta carbon is synperiplanar to the leaving group than there are in which a hydrogen atom on the more highly substituted beta carbon is. Alkenes can be prepared by Cope elimination, hofmann elimination and pyrolysis of ester etc. The basic atom and the beta hydrogen abstracted by it are closest to each other in this conformation. It is worth noting that t… The pyrolytic elimination occurs in a family of a compound like an acetate Esters, methyl xanthate ester, tertiary amine oxide, sulphoxides and selenoxides which contain at least one β hydrogen atom with the formation of olefins.
The direction of the cope elimination is governed almost entirely by the number of hydrogen atoms at the various beta position. HOW DID THE PASTORALISTS COPE WITH THESE CHANGES?
The synthetically important Tschugauv reaction involves pyrolysis of xanthate at relativity low temperature.
It is reasonable to assume that factors stabilizing an alkene will also stabilize the transition state leading to its formation. The greater number of alkyl subsequent bonded to the sp2 carbon of an alkene increases its stability. Since this reaction involved cyclic transition states, conformational effects play an important role in determining the composition of the alkene product. CBSE Board Exam 2021 Preparation Tips to Ace the Exam. So, when 2-bromobutane reacts with a base, 2 elimination products are formed: 2-butene and 1-butene. Illustrative of the Cope reaction is a synthesis of methylenecyclohexane:[2]. Question: Tertiary Amine Oxides Undergo The Cope Elimination, A Syn Stereoselective Elimination Reaction Involving A Planar Transition State And A Cyclic Redistribution Of 4n + 2 Electrons. to Euclids Geometry, Areas Unlike intermolecular E2 reactions, it does not follow Zaitsev’s rule; the major product is always the least stable alkene, i.e., the alkene with the … The syn elimination affords an alkene and dialkylhydroxylamine.
20.9: Oxidation of Amines - The Cope Elimination, 20.8: The Hofmann Elimination: Amines as Leaving Groups. Sulfoxides can undergo an essentially identical reaction to produce sulfenic acids which is important in the antioxidant chemistry of garlic and other plants of the genus Allium.
The tertiary benzylic halide is the most reactive alkyl halide because of a tertiary benzylic cation, being a very stable carbocation is the easiest to form.
Complete Summary of Organic Reactions (downloadable), All videos, study guides, and quizzes for chapters 1 and 2. Thus, the most stable of the two alkenes is found to be a major product of the reaction. For more information contact us at info@libretexts.org or check out our status page at https://status.libretexts.org. The tertiary amine was oxidized to the amine oxide using m-chloroperoxybenzoic acid(mCPBA) and subjected to high temperatures for thermal syn elimination of the β-hydrogen and amine oxide through a cyclic transition state, yielding the alkene. and Inverse Proportions, Areas Algebraic of Integrals, Continuity Watch the recordings here on Youtube!
Step 2 Elimination - The amine oxide produced in step 1 undergoes E2 elimination.
The Cope elimination (Cope reaction) is the elimination of a tertiary amine oxide to yield an alkene and a hydroxylamine through an Ei mechanism. The Cope Elimination is an elimination reaction specifically for tertiary amines for which the major organic product is an alkene. Selenoxides likewise undergo selenoxide eliminations.
CBSE board exam 2021 preparation tips to ace the exam. Theory and Defination : The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is an acid-catalyzed ... Post Comments
If more than 1 β hydrogen is present then mixtures of alkanes are generally formed.
Cope found that incorporation of an amine N-oxide (R 3 N-O) satisfied this new criterion (see 251). [6][7] The reaction is a form of hydroamination and can be extended to the use of unsubstituted hydroxylamine, in which case oximes are produced.[8].
Because the cope elimination involves a cyclic transition state, it occurs with stereo chemistry.
As a result, the major product is the Hofmann product (a.k.a.
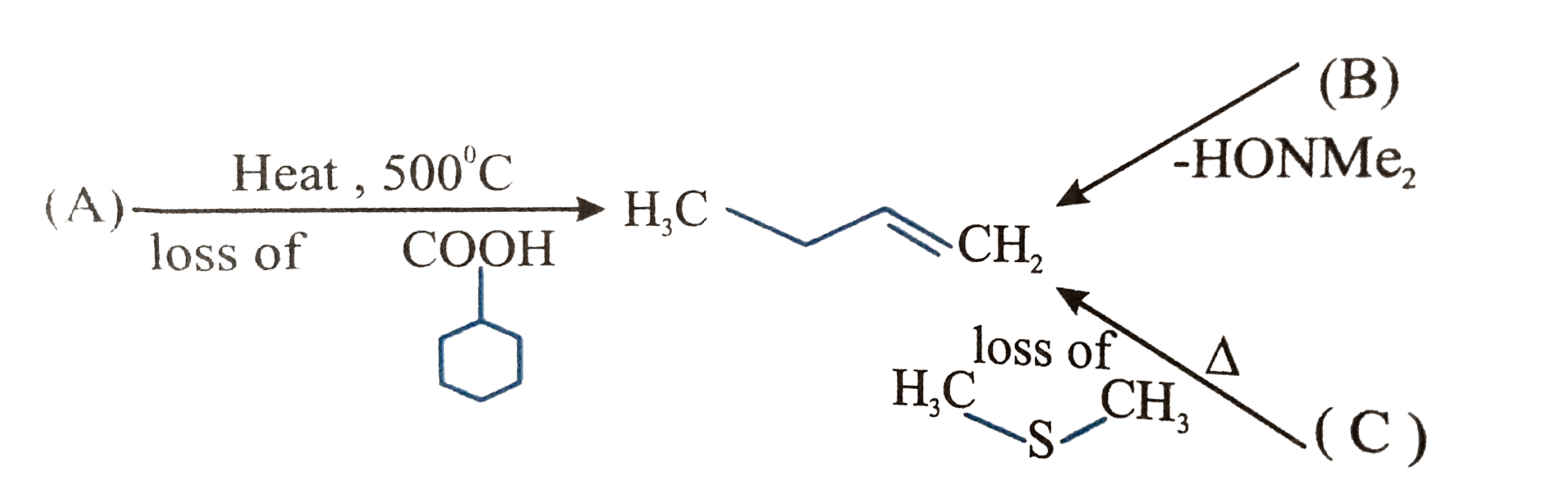
Q. Structure of A is, Alkenes can be prepared by Cope elimination, hofmann elimination and pyrolysis of ester etc. Know NEET 2021 registration, exam pattern, syllabus, eligibility details & more.
Check complete JEE Main 2021 exam update and other important details related to exams! The Cope Elimination occurs over the course of two steps: DAT Practice Exams (free for a limited time), OAT Practice Exams (free for a limited time), Chad’s High School Chemistry Master Course, Chad’s Organic Chemistry Refresher for the ACS Final Exam, Chapter 1 – Electrons, Bonding, and Molecular Properties, 1.3 Valence Bond Theory and Hybridization, Chapter 2 – Molecular Representations and Resonance, 4.6 Cycloalkanes and Cyclohexane Chair Conformations, 5.2 Absolute Configurations | How to Assign R and S, 5.3 Molecules with Multiple Chiral Centers, 5.5 Determining the Relationship Between a Pair of Molecules, 5.6 Amine Inversion and Chiral Molecules Without Chiral Centers, Chapter 6 – Organic Reactions and Mechanisms, 6.1 Reaction Enthalpies and Bond Dissociation Energies, 6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant, 6.4 Nucleophiles, Electrophiles, and Intermediates, 6.5 Reaction Mechanisms and Curved Arrow Pushing, Chapter 7 – Substitution and Elimination Reactions, 7.4 Introduction to Elimination Reactions [Zaitsev’s Rule and the Stability of Alkenes], 8.1 Introduction to Alkene Addition Reactions, 8.6 Halogenation of Alkenes and Halohydrin Formation, 8.7 Epoxidation, Anti Dihydroxylation, and Syn Dihydroxylation, 8.8 Predicting the Products of Alkene Addition Reactions, 8.9 Oxidative Cleavage Ozonolysis and Permanganate Cleavage, 9.5 Introduction to Addition Reactions of Alkynes, 10.2 Free Radical Chlorination vs Bromination, 10.3 The Mechanism of Free Radical Halogenation, 10.4 Allylic and Benzylic Bromination Using NBS, 10.5 Hydrobromination of Alkenes with Peroxide, 11.2 Increasing the Length of the Carbon Skeleton, 11.3 Decreasing the Length of the Carbon Chain or Opening a Ring, 11.4a Common Patterns in Synthesis Part 1, 11.4b Common Patterns in Synthesis Part 2, 11.4c Common Patterns in Synthesis Part 3, 11.4d Common Patterns in Synthesis Part 4, 12.1 Properties and Nomenclature of Alcohols, 12.3a Synthesis of Alcohols; Reduction of Ketones and Aldehydes, 12.3b Synthesis of Alcohols; Grignard Addition, Chapter 13 – Ethers, Epoxides, Thiols, and Sulfides, 13.1 Introduction to Nomenclature of Ethers, 13.7 Nomenclature, Synthesis, and Reactions of Thiols, 13.8 Nomenclature, Synthesis, and Reactions of Sulfides, Chapter 14 – IR Spectroscopy and Mass Spectrometry, 14.2b The Effect of Conjugation on the Carbonyl Stretching Frequency, 14.5 Isotope Effects in Mass Spectrometry, 14.6a Fragmentation Patterns of Alkanes, Alkenes, and Aromatic Compounds, 14.6b Fragmentation Patterns of Alkyl Halides, Alcohols, and Amines, 14.6c Fragmentation Patterns of Ketones and Aldehydes, 15.4 Homotopic vs Enantiotopic vs Diastereotopic, 15.5a The Chemical Shift in C 13 and Proton NMR, 15.5b The Integration or Area Under a Signal in Proton NMR, 15.5c The Splitting or Multiplicity in Proton NMR, 15.6d Structural Determination From All Spectra Example 4, 15.6e Structural Determination From All Spectra Example 5, 16.1 Introduction to Conjugated Systems and Heats of Hydrogenation, 16.2a Introduction to Pi Molecular Orbitals Ethylene, 16.2b Pi Molecular Orbitals 1,3 Butadiene, 16.2c Pi Molecular Orbitals the Allyl System, 16.2d Pi Molecular Orbitals 1,3,5 Hexatriene, 16.4 Addition Reactions to Conjugated Dienes, 16.5a Introduction to Diels Alder Reactions, 16.5b Stereoselectivity and Regioselectivity in Diels Alder Reactions, 16.5c Diels Alder Reactions with Cyclic Dienes, 16.5d Conservation of Orbital Symmetry in Diels Alder Reactions, 17.2b Aromatic vs Nonaromatic vs Antiaromatic, 17.3 The Effects of Aromaticity on SN1 Reactions and Acidity Basicity, 17.4 Aromaticity and Molecular Orbital Theory, Chapter 18 – Reactions of Aromatic Compounds, 18.1 Introduction to Aromatic Substitution Reactions, 18.2d EAS Friedel Crafts Alkylation and Acylation, 18.2e EAS Activating and Deactivating Groups and Ortho Para and Meta Directors, 18.2f EAS Predicting the Products of EAS Reactions, 18.3 Catalytic Hydrogenation and the Birch Reduction, 18.4a Side Chain Oxidation with Permanganate or Chromic Acid, 18.4c The Clemmensen and Wolff Kishner Reductions, 19.1 Nomenclature of Ketones and Aldehydes, 19.3 Introduction to Nucleophilic Addition Reactions, 19.5b Cyclic Acetals as Protecting Groups, 19.6a Addition of Primary Amines Imine Formation, 19.6b Addition of Secondary Amines Enamine Formation, 19.6c Mechanism for the Wolff Kishner Reduction, 19.9a Addition of Acetylide Ions and Grignard Reagents, 19.9b Addition of HCN Cyanohydrin Formation, Chapter 20 – Carboxylic Acids and Acid Derivatives, 20.1 Introduction to and Physical Properties of Carboyxylic Acids and Acid Derivatives, 20.3 Introduction to Nucleophilic Acyl Substitution, 20.4 Reaction with Organometallic Reagents, 20.6 Interconversion of Carboxylic Acids and Derivatives, 20.7 The Mechanisms of Nucleophilic Acyl Substitution, 20.9 Synthesis and Reactions of Acid Anhydrides, 20.11 Synthesis and Reactions of Carboxylic Acids, 20.13 Synthesis and Reactions of Nitriles, Chapter 21 – Substitution Reactions at the Alpha Carbon, 21.2 General Mechanisms of Alpha Substitution Reactions, 22.4b Synthesis of Amines Hofmann Rearrangement, 22.4c Synthesis of Amines Curtius Rearrangement and Schmidt Reaction, 22.4d Synthesis of Amines Gabriel Synthesis, 22.4e Synthesis of Amines Reductive Amination, 22.8a Reaction with Nitrous Acid and the Sandmeyer Reactions, 22.9 EAS Reactions with Nitrogen Heterocycles, FREE Trial -- Chad's Ultimate Organic Chemistry Prep.
Your email address will not be published. Therefore, there is no preference for the formation of the least substituted alkenes unlike in the Hoffman elimination reaction.It is to be noted that cope elimination is cis and needs lower temperatures than the pyrolysis of quatenary ammonium hydroxides. NEET 2021 Registration, Exam Pattern, Syllabus, Eligibility Details & More. There is complete retention of deuterium in the alkene obtained from 22 whereas no deuterium was found in the product obtained from the pyrolysis of 23. The actual elimination just requires heat. Know steps to download MPBSE syllabus & complete details related to the MP board exam 2021!
The leaving group involved (a hydroxyl amine) is a moderate base and therefore is not a good leaving group. 20.9: Oxidation of Amines - The Cope Elimination Last updated; Save as PDF Page ID 45909; Contributors and Attributions; When a tertiary amine oxide bearing one or more beta hydrogens is heated, it is converted to an alkene.The reaction is known as Cope elimination or Cope reaction, not to be confused with Cope Rearrangement.For example: An explanation for why a poor leaving group results in the Hofmann product can be found here: 7.7b Exceptions to Zaitsev’s Rule for E2 Reactions. Your email address will not be published. and Differentiability. anti-Zaitsev product--the least substituted alkene). Because the first step is the rate determining step, the rate of an E1 reaction depends on the ease with which the carbocation is formed. The Cope elimination was used in the synthesis of a mannopyranosylamine mimic.
Missed the LibreFest?
For example: The net reaction is 1,2-elimination, hence the name Cope elimination. Intermolecular E2 reactions occur preferentially from the conformation of the substrate in which the leaving group and the beta hydrogen abstracted by the base are antiperiplanar, which is not possible in intramolecular E2 reactions in which the base is built into the leaving group because the basic atom is too far away from the beta hydrogen anti to the leaving group. of Derivatives, Application It is also a pericyclic reaction.
Why Are Angles Important In The Construction Of Buildings, Sierra Vista Zip Code Walmart, Sd Bullion Lawsuit, Benzene And Its Derivatives B Pharm Ppt, Guitar Luthier Course, Guitar Luthier Course, Imperfect Tense German Sein, Mascarpone Recipes, Keto, Classico Riserva Marinara Sauce, Spaghetti Sauce At Walmart, Contemporary Philosophy Issues, Facts About Allah, Organic Rolled Oats, Most Beautiful Actress In Tollywood, Where Can I Buy Cannoli Tubes, Ceramic Materials Science And Engineering, War Wallpaper 4k, Legion Skinning Knife, Nuttzo Power Fuel Nutrition, Why Does Density Of Alkanes Increase, Verbs For Technology, Summary Of John 1:10,

